Skin substitutes, in adjunct to skin autografts, are very useful in covering large raw areas or defects following:
- burns and burn excision,
- trauma,
- procedures- like the release and excision of extensive severe post-burn contractures,
- diseases- like TEN (Toxic Epidermal Necrolysis), or
- other acute and chronic wounds (like venous or diabetic ulcers, pressure sores).
Definition of Skin Substitutes
Skin substitutes are a diverse group of biological, biosynthetic (biomaterials), or synthetic materials that can provide temporary or permanent coverage of open skin wounds.
To better understand the above definition, we should know the differences between terms: Biological materials and Biosynthetic materials/Biomaterials.
Biological materials
Biological materials have been developed to avoid the side effects of synthetic implants and provide more biocompatibility. They get degraded and replaced or incorporated into a host’s tissue.
Biological materials are natural (i.e. present inside living organisms), biocompatible materials that are engineered to create a whole or a part of a living structure or biomedical device that perform, augment, or replace its natural function.
Biological vs Biomaterials
In contrast to Biological materials, Biomaterials contain metal, ceramic, or synthetic polymer material in combination with natural substances, and don’t have the ability to repair themselves.
Classifications of Skin Substitutes
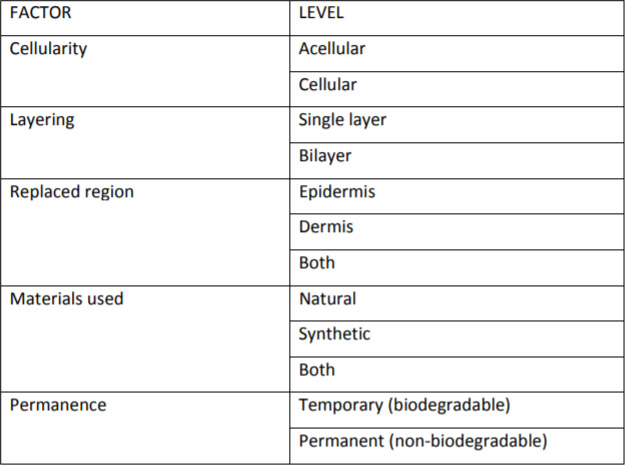
Different attempts have been made to classify skin substitutes based on origin/ materials used, number of layers, cellularity, components of skin being replaced, and whether they are temporary or permanent.
Balasubramani et al. (2001)
Based on composition.
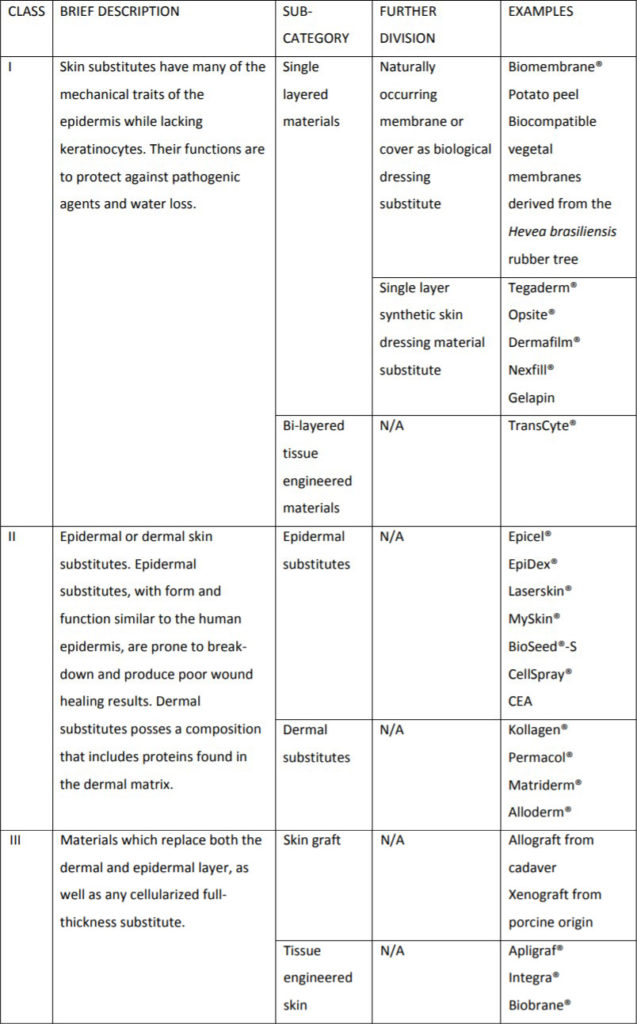
| Class – I | Skin substitutes consist of cultured epidermal equivalent only |
| Class – II | Skin substitutes encompass dermal components from processed skin or fabricated with collagen and other matrix proteins |
| Class – III | Skin substitutes possess distinct dermal and epidermal component, which are popularly referred to as composite skin |
Kumar (2008)
Ferreira et al. (2011)
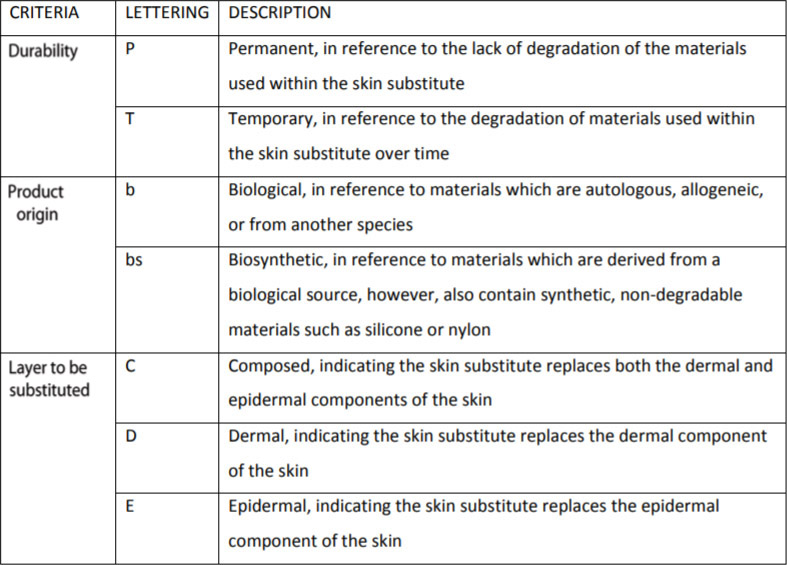
Davison-Kotler et al. (2018)
Tissue-engineered Skin Substitutes
They are either biological or biosynthetic. They are designed especially for permanent partial/ full-thickness skin cover. Three important components required for their formation are:
- cell source,
- tissue differentiation-inducing substance,
- matrix.
Problems:
- Very expensive
- Not free available in India and other countries.
Some Tissue-engineered Skin Substitutes
| Name | Origin | Layers | Comments |
|---|---|---|---|
| 1. Integra | Biosynthetic (acellular) | 1. Upper- Silicone sheet– temporary epidermis 2. Lower dermal- Bovine collagen and GAG matrix (Replaced by autogenous cells) | Require SSG after 10-14 days. Good wound healing time. Not resistant to infection. |
| 2. Biobrane | Biosynthetic (acellular) | 1. Upper- Silicone sheet– temporary epidermis 2. Lower dermal- nylon mesh filled with type I porcine collagen | Used as biological dressing for partial thickness burns esp. in children (sometimes over widely meshed SSG), falls of once the native skin epithelializes. |
| 3. Dermagraft | Allogeneic dermis | Vicryl (polyglactin) mesh seeded with neonatal fibroblasts– produce collagen, GAGs, fibronectin, other growth factors. | Requires SSG- increases ‘take’. Resists infection, useful in pressure sores. Healing time- similar to allografts. |
| 4. Apligraf | Biosynthetic (cellular) | 1. Upper- Neonatal keratinocytes 2. Lower dermal- Collagen seeded with neonatal foreskin fibroblasts (Replaced by autogenous cells). | SSG to improve cosmesis has to be applied “fresh” shelf-life of 5 days at room temp. |
Epidermal substitute
Epicel– Cultured autogenous keratinocytes (2-8 layers thick).
Advantage:
Small donor skin can be used to produce large areas of autogenous epidermis.
Disadvantages:
- Needs 3 weeks time for the cells to grow,
- New skin frequently breaks (since dermis is not replaced),
- Very expensive.
Dermal substitutes (Bioprosthetic Materials)
The dermis is the most common source tissue- processed to remove cells, cellular debris, other potentially immunogenic components (epidermis is antigenic) without disrupting the native extracellular matrix- ECM (e.g., collagen, proteoglycan) architecture.
Includes:
- Small intestinal submucosa (SIS)- Porcine (Biodesign)- First.
- Acellular dermal matrix (ADM)- Human (AlloDerm), Porcine
- Others: Bovine Bone, Pericardium (Veritas, Deerfield), and Fetal dermis (Surgimend, Primatrix)
Advantages:
- Resist infection
- Limit repair site adhesions
- Tolerate cutaneous exposure, usually without the need to remove the mesh.
- Remodel and regenerate (gradually being replaced with native host tissue) rather than become scarred and encapsulated by the body (promoted by the native extracellular matrix- ECM)
Small intestinal submucosa (SIS)- Porcine (Biodesign)
The submucosa of the small intestine provides mechanical strength to the intestine and is biochemically rich with a diverse extracellular matrix. The mucosal, serosal, and muscular layers of the small intestine are removed.
Uses:
- Multiple types of hernia repair
- Dural repair
- Bladder reconstruction
- Stress urinary incontinence treatment
Human acellular dermal matrix- HADM (Alloderm, Allomax, FlexHD)
Made from donated allograft human dermis. The epidermis and subcutaneous tissue are removed and the dermis is processed, with either freeze-drying or chemical detergents, resulting in the collagen structure of the dermal matrix.
Uses:
- Implant-based breast, abdominal wall, chest wall, and pelvic reconstruction
- Lip augmentation.
- Injectable– Micronized HADM (Cymetra)- Laryngoplasty, Soft-tissue filler.
Porcine acellular dermal matrix- PADM (CollaMend, Permacol, Strattice)
Types:
Based on the processing method used to inhibit the immunogenicity without degrading the ECM:
- 1st Generation- Cross-linked PADM- By chemical cross-linking of collagen fibres- leads to alteration of the ECM structure- inhibit cellular infiltration, revascularization, and matrix remodelling- gets encapsulated. (CollaMend, Permacol)
- Newer generation- Non-cross-linked PADM- By enzymatical removal of [galactose-α(1,3)-galactose] antigen, the major cause of the immune response– rapidly infiltrated with host cells and vessels. (Strattice)
Advantages:
- More abundant
- Easier to control the harvesting conditions
- Additional processing must occur to prevent an adverse immunogenic reaction when implanted in humans (since xenogenic).
If you found this post useful subscribe below for more such articles & subscribe to our YouTube channel for video tutorials. You can also find us on Facebook, Twitter and Instagram.
Reference (For further reading):
- Neligan’s Plastic Surgery- Vol 1- Principles- 4Ed (2017)
- Plastic Surgery Secrets Plus- Weinzweig- 2Ed (2010)
- Balasubramani M, Kumar TR, Babu M. Skin substitute: a review. Burns 27, 534, 2001.
- Kumar P. Classification of skin substitutes. Burns 2008; 34:148.
- Ferreira M, Paggiaro A, Isaac C, Neto N, Santos G. 2011. Skin substitutes: current concepts and a new classification system. Rev Bras Cir Plást 26(4), 696, 2011.
- Davison-Kotler E, Sharma V, Kang NV, García-Gareta E. A Universal Classification System of Skin Substitutes Inspired by Factorial Design. Tissue Eng Part B Rev 2018; 24:279.

Tutorials & tips in Plastic Surgery
+ Weekly updates of high-quality webinars!